
Assistant Professor
E-mail: kinshuk at rcb dot res dot in
Rapidly growing implementation of enzymes for the production of a diverse range of high-value chemicals is driven by an enzyme’s unique ability to operate under a mild and aqueous condition with high selectivityand specificity. Therefore, incorporation of enzymes into existing synthetic manufacturing routes results in a significant reduction in the production cost and hazardous waste. Despite of significant advantage of the enzyme-based process, only a small portion of enzymes have been exploited in the industry due to unavailability of enzymes which can catalyze desired chemical transformation or the instability of available enzymes in industrially harsh conditions. The emerging economy and the merit of enzyme-based process demands researchers to discover and engineer enzymes to evolve superior biocatalysts with desired reactivity, specificity, and stability required to catalyze wide-range of chemical transformations.
Our research focus is to harness powerful chemical reactivity and selectivity of biosynthetic enzymes and engineer their properties to evolve superior biocatalyst, as well as to develop a biocatalytic process for the production of high-value chemicals such as pharmaceutical compounds, smart biomaterials and biofuel.
For the studies, my group will be developing fundamental new knowledge about enzymes’s structure, stability, kinetic activity, regio- and stereo-selectivity, substrate,and cofactor binding specificity, solvation and conformational dynamics in carrying out key chemical transformations. The knowledge gained will be utilized to engineer enzyme candidates, following rational and semi-rational approach, to develop superior biocatalyst with novel and improved properties required for their industrial-scale implementation to produce high-value chemicals.
The overview ofenzyme discovery and engineering approach to evolve biocatalyst with novel and improved properties.
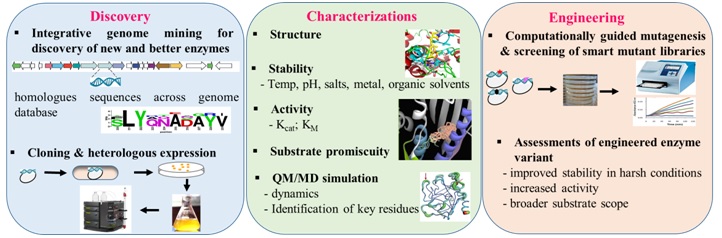
The far-reaching objectives are as follows
A. Discovery and engineering of enzymes for biocatalytic synthesis of pharmaceuticals and pharmaceutical intermediates.
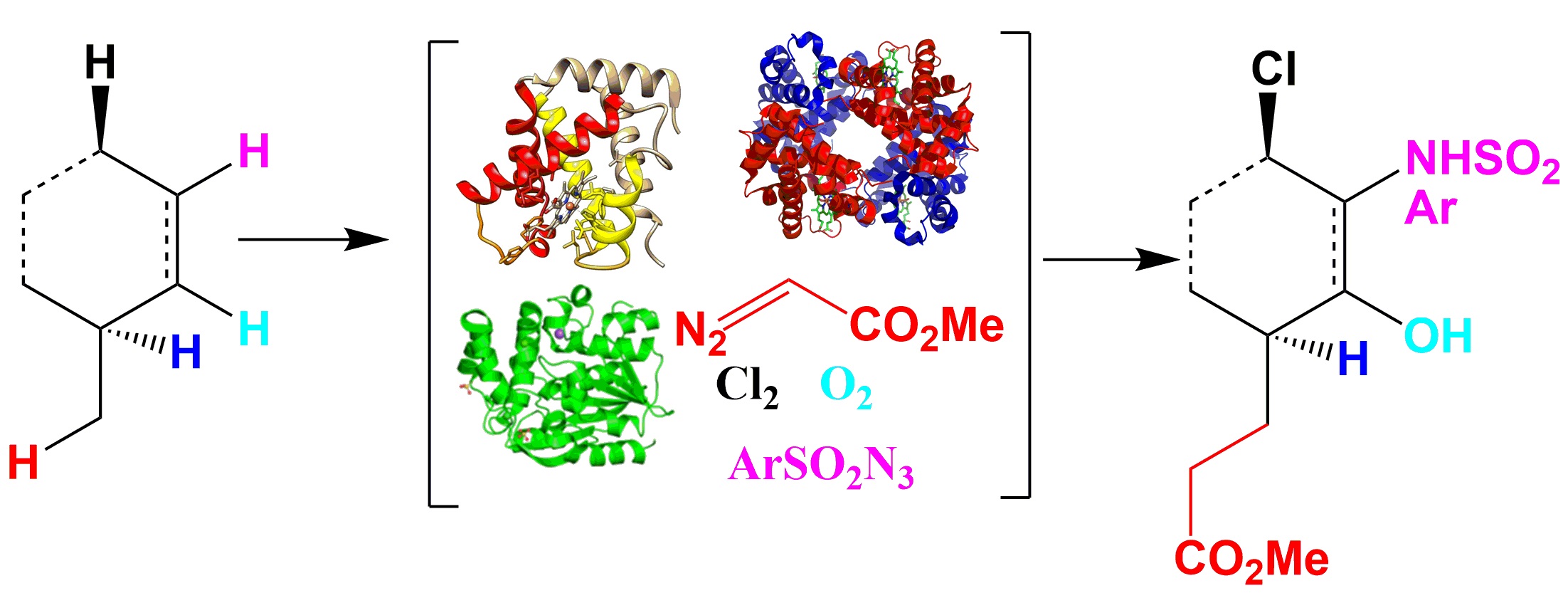
Potential enzyme classes: P450 enzymes, halogenases, transaminases,transerases, cyclases etc.
B. Biocatalytic conversion of polymeric materials (plastic waste, lignocellulosic feedstocks) to energy, fuel, and chemicals.
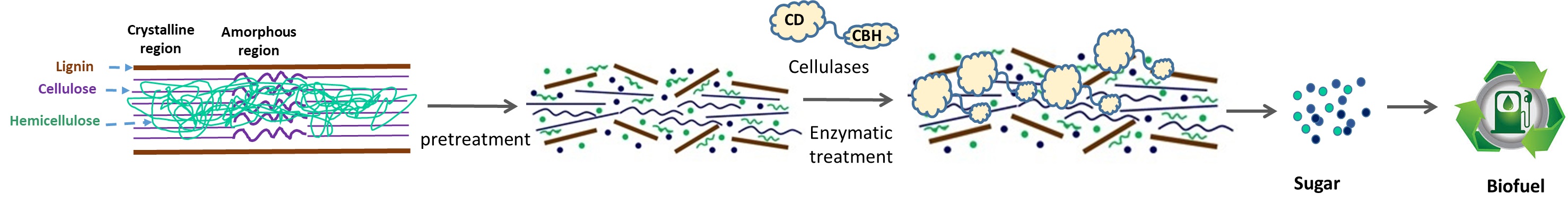
Please come back later for further details about the specific projects actively running in the lab.
The research projects are interdisciplinary in nature, therefore, there are opportunities for rigorous training in cutting-edge chemistry, molecular biology, biochemical and biophysical techniques. This learning will uniquely prepare students and postdoc scholars for a career in academia, chemical, pharmaceutical and biotechnology industries, education, and a variety of other fields.
Motivated masters and graduate students, visiting scholars and postdoctoral researchers with various backgrounds including chemistry, biochemistry, biophysics, biotechnology, bioinformatics, and computational biology are welcome to join our group.
Patel, K; Srivastava, K. R.;Durani, S. Zinc-finger hydrolase: Computational selection of linker and sequence towards metal activation with protein. Bioorg. Med. Chem.,2010, 18, 8270-8276.
Srivastava, K. R.; Kumar, A.; Durani, S. “Stereochemistry and solvent role in protein folding: Nuclear magnetic resonance and molecular dynamics studies poly-L and alternating-L,Dhomopolypeptides in DMSO.” J. Phys. Chem. B, 2011,115, 6700-6708.
Srivastava, K. R.; Kumar, A.; Durani, S. “Stereochemistry and protein folding: Spectroscopic and molecular dynamics studies of homopolypeptides in DMSO” Biophys. J.,2011,100, 3a.
Srivastava, K. R.;Majumder, R.; Quinn-Allen, M. A.; Kane, W. H.; Lentz, B. R. Phosphatidylserine and FVa regulate FXa structure. Biochem. J., 2014,459, 229-239.
Srivastava, K. R. (corresponding author);Durani, S. Interactions of main chain in folding and self-assembly of unfolded protein structure: Enquiries with a serine solubilized nonapeptide.AIP Advances,2014, 4, 067140.
Srivastava, K. R. (corresponding author);Durani, S. A Stereochemically bent beta-Hairpin: scrutiny of driving forcesfolding proteins by critically comparing heteropolypeptide and cognate oligoalanine. O. J. Phys. Chem.,2014,4, 81-97.
Srivastava, K. R. (corresponding author);Durani, S. Design of zinc-finger hydrolase with synthetic αββ protein. PLoS ONE,2014, 9, e96234.
Goyal, B.; Patel, K; Srivastava, K. R.;Durani, S. De novo design of stereochemically-bent sixteen-residue -hairpin as a hydrolase mimic. RSC Advances,2015,5, 105400-105408.
Srivastava, K. R.; Lapidus, L. J. Aggregation propensity of prion is kinetically controlled by intramolecular diffusion of protein chain. Biophys. J., 2015,108, 46a.
Srivastava, K. R.; French, K. C.; Tzul F. O., Makhatadze, G. I.; Lapidus, L. J. Intramolecular diffusion controls aggregation of the PAPf39 peptide. Biophys. Chem.,2016,216, 37-43.
Acharya, S.; Srivastava, K. R.; Lapidus, L. Monomer dynamics of Aβ and kinetic control of early aggregation in alzheimer’s disease. ChemPhysChem,2016,17, 3470-3479.
Goyal, B.; Srivastava, K. R.;Durani, S. Examination of the effect of N-terminal diproline and charged side chains on the stabilization of helical conformation in alanine–based short peptides: A molecular dynamics study.Chemistry Select,2016,1,6321-6327.
Goyal, B.; Kumar, A; Srivastava, K. R.;Durani, S. Computational scrutiny of the effect of N-terminal proline and residue stereochemistry in the nucleation of α-helix fold.RSC Advances,2016,6, 74162-74176.
Goyal, B.; Srivastava, K. R.;Kumar, A., Patwari, G. N. Durani, S. Probing the role of electrostatics of polypeptide main-chain in protein folding by perturbing N-terminal residue stereochemistry: DFT study with oligoalanine models.RSC Advances,2016,6, 113611-113619.
Goyal, B.; Srivastava, K. R.; Patel, K; Durani, S. Modulation of β-hairpin peptide self-assembly: A twenty-residue poly-L β-hairpin.Chemistry Select,2016,1,2050-2057.
Goyal, B.; Kumar, A; Srivastava, K. R.;Durani, S. Scrutiny of chain-length and N-terminal effects in α-helix folding: A molecular dynamics study on polyalanine peptides.J. Biomol. Struct. Dyn.,2017, 35,1923-1935.
Goyal, B.; Srivastava, K. R.;Durani, S. N-terminal diproline and charge group effects on the stabilization of helical conformation in alanine-based short peptides: CD studies with water and methanol as solventJ. Pep. Sci.,2017, 23, 431–437.
Srivastava, K. R. (corresponding author);Goyal, B, Kumar, A.; Durani, S. Scrutiny of Sequence Role in Protein Folding by Comparing Heteropolypeptides and Cognate Polyalanines: A Study with DMSO as Model Solvent. RSC Advances2017,7, 27981-27991.
Collier, T. J. ( Corresponding & co-first Author);Srivastava, K. R. (co-first Author);Justman, C.; Grammatopoulous, T.; Hutter-Paier, B.; Prokesh, M.; Havas, D.; Rochet, Jean-Christophe, Liu, F.; Jock, K.; Oliveira, P.; Stirtz, G. L.; Dettmer, U.; Sortwell, C. E; Feany, M. B.; Lansbury, P.; Lapidus, L. J.; Paumier, K. L. “Nortriptyline inhibits aggregation and neurotoxicity of α-synuclein by enhancing reconfiguration of the monomeric form.Neurobiol of Disease,2017,106, 191-204.(Research appeared in several science & clinical news portal)
Srivastava, K. R.; Lapidus, L. “Prion protein dynamics before aggregation” Proc. Nat. Ac. Sci. 2017, 114, 3572–3577.(Research appeared in several news portal across USA)
Srivastava, A.; Srivastava, K. R.; Hebber, M.; Su, F., Cao, X., Chinnaiyan, A. Girisha, K. M., Shukla, A., Bielas, S. L. Genetic diversity of NDUFV1-dependent Mitochondrial complex I deficiency.Eur. J. Hum. Genet,2018, 26,1582-1587.
Woodard, J. C.; Srivastava, K. R.; Rahamim, G.; Grupi, A.; Hogan, S.; Nawrocki, G.; Haas, E.; Feig, F.; Lapidus, L. J. “Intramolecular Diffusion in α-Synuclein: It Depends on How You Measure It” Biophy. J., 2018, 115, 1190-1199.
Kinshuk Raj Srivastava
Regional Centre for Biotechnology
NCR Biotech Science Cluster
3rd Milestone, Faridabad-Gurgaon Expressway
P.O. Box No. 3, Faridabad - 121 001
Haryana (NCR Delhi), India
E-mail: kinshuk at rcb dot res dot in
Phone: 91 129-2848835